STEP-46 Project
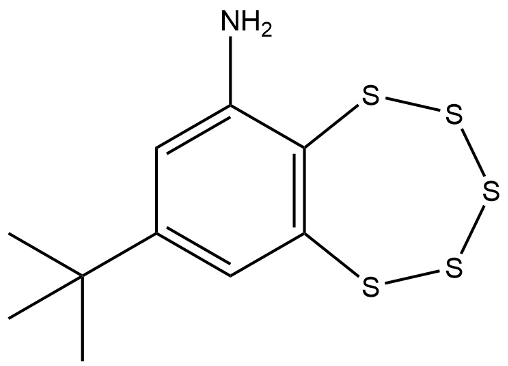
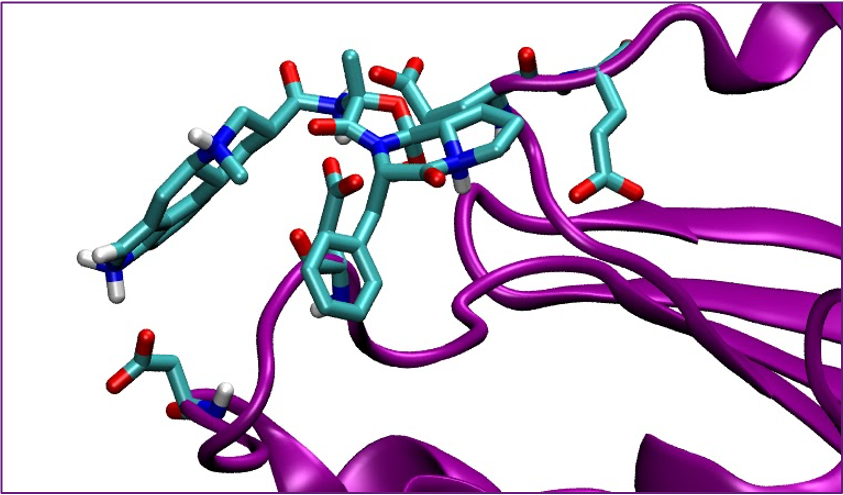
Understanding the negative modulation of neuroplasticity is key to developing treatments for neurodegenerative disease. Recent research has identified a brain specific enzyme called striatal enriched tyrosine phosphatase (STEP) as a potential target for drug design. STEP has a multitude of natural substrates that are connected to synaptic strengthening such as N-methyl-d-aspartate receptors (NMDARs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), and many other proteins and enzymes. Several STEP inhibitors have been identified but only one has been shown to be effective, 8-(trifluoromethyl)-1,2,3,4,5-benzopentathiepin-6-amine hydrochloride (TC-2153). TC-2153 is rather expensive, $3900 per gram, due in part to its complex polysulfide structure and has been identified as an environmental hazard. However, TC-2153’s IC50 is 24.6 nM, making it many times more potent than any other STEP inhibitor discovered so far. The highly conserved nature of phosphatase active sites makes the design of selective competitive inhibitors difficult. Hou, et al, in 2019 published a paper identifying three cryptic pockets that could be exploited as druggable sites on the surface of STEP.
The low druggability of STEP makes it the ideal candidate for early computational discovery. Using computational screening, several million ligands can be assessed for binding affinity. This study used Autodock Vina to compare the docking score of TC-2153 to other potential inhibitors. Molecular dynamics and other more precise measures will also be performed to further screen and characterize the most promising drugs in hopes of developing a novel, potent inhibitor of STEP.
Current Members